Success Stories
Türkiye Saving 1 Billion US Dollars Annually with ‘İTS’!
In recent years, the pharmaceutical distribution and tracking landscape has been radically transformed, with Türkiye leading the charge by pioneering the world's first end-to-end drug track and trace system. This system, known as DrugXafe, not only enhances the security and transparency of the drug supply chain but also translates into significant economic benefits for the nation.
Pharma counterfeiting is a significant problem worldwide and over the last few years, the number of counterfeit drugs has increased. Ongoing education of the public and stricter laws governing the control of the products so far are making little impact in combating the issue. As counterfeiters become more sophisticated, pharmaceutical manufacturers and their trading partners need to improve the safety and security of the pharmaceutical supply chain. In Türkiye, all stakeholders have licenses issued by the Turkish Medicines and Medical Devices Agency (TMMDA). The data is registered in the ITS system after obtaining the TMMDA scientific committee’s permission. The Agency also defines the price of a drug; 91 % of medicines in Türkiye are paid for through the reimbursement association of the state, making the state the biggest customer of the pharmaceutical industry in Türkiye.
Challenge
The main challenge in Türkiye was to ensure and guarantee the reliable supply of drugs to patients. Türkiye's drug supply, like most countries, was at risk of illegal activities that could seriously impact public health and safety, ranging from theft and diversion of legitimate drugs to counterfeiting. Türkiye needed to find ways to prevent the sale of illegal drugs, smuggled drugs or counterfeit drugs and fight reimbursement fraud, among other threats to the safety and security of the supply chain.
Solution
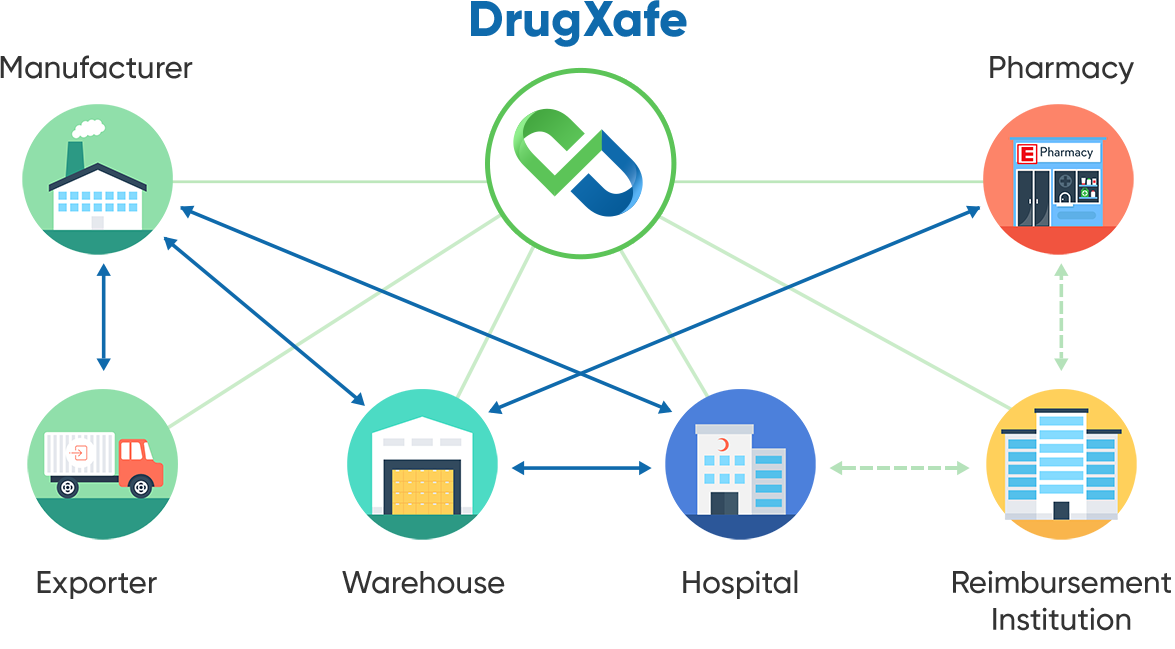
The solution is traceability, defined as complete, end-to-end, actionable visibility of finished pharmaceuticals in healthcare globally, from the point of production to the point of use. That is why Türkiye developed its own Pharmaceutical Track and Trace System, ITS. In addition, Türkiye built a centralized repository to provide a single location that all the stakeholders must use to notify all activities related to drug movement through the supply chain. With such a central management system, the ITS can track and trace a drug using its serial number from the point of manufacture to the point of dispense. By serving as the single source of accurate data in the pharmaceutical supply chain, ITS has become a fully interoperable, electronic, serialisation-based track & trace system for pharmaceuticals in Türkiye and can serve as a model for other countries looking to establish a similar model.
PTS and ITS
ITS is limited to tracking and tracing the secondary level of packaging of the drug, as the majority of drugs sold, bought and transported are done via cartons, boxes and palettes. Türkiye developed the Package Transfer Service (PTS), a centralized file-sharing platform to track and trace the shipping level. PTS provides an electronic language (PTS XML) that stakeholders can use to share the data related to their shipment and exchange. The manufacturer produces the finished goods, uniquely identifies them and includes a GS1 DataMatrix containing additional information: the Global Trade Item Number (GTIN), expiration date, serial number and batch/lot number.
The goods are then packed in boxes labeled with a barcode, which could be a GS1 barcode. After the shipment is prepared, the manufacturer sends a "manufacture notification" message to ITS and creates an XML file containing pertinent data for the units in the container; this data is then uploaded to the PTS via an XML file. This is the first record that begins the CoC/CoO of the drug pack. After the shipment is delivered, the wholesaler downloads the XML file from PTS, scans it to verify that it matches the shipment received and sends the purchase action to ITS. The ownership of the drug then changes from the manufacturer to the wholesaler.
The retail pharmacy or hospital follows the same procedure after sending their order. The wholesaler sends the sale notification to ITS and uploads the XML file to the PTS. The buyer (retail pharmacy or hospital) downloads the XML file from PTS and uses the information from the drug packs to notify ITS of the purchase action. For hospitals, the consumption notification is done right before opening the drug pack, which symbolizes the end of the product's "life" and is marked as such in the system. The pedigree ends at this stage, which prevents this drug from re-entering the supply chain. In the retail pharmacy setting, after getting the patient's prescription, the pharmacy provides the drug pack to the patient. It electronically sends the invoice to the reimbursement association. The reimbursement association queries the sale from ITS and verifies that the information sent by the retail pharmacy is correct before reimbursing the retail pharmacy.
Benefits
Türkiye has more than 40,000 stakeholders included in the ITS system and has tracked and traced more than 10 billion drug packs. Daily, there are more than 45 million transactions through the system. The system response time is 0,02 seconds per transaction and its performance record is outstanding, with an uptime ratio of 99,999%! Türkiye's main goal was to ensure the reliable and safe supply of drugs to patients. By implementing a traceability system, Türkiye has achieved that and more.
The system has contributed to Türkiye's ability to combat the sale of counterfeit and smuggled drugs, preventing illegal sale of drugs, barcode scams and drug thefts. The results of Türkiye's efforts have been tremendous and in these five areas alone, the nation is seeing savings of 1 billion US dollars annually. This is a significant saving considering that pharmaceuticals are a ten billion dollar annual industry in Türkiye. In addition, the electronic system has streamlined supply chain processes, saving time and increasing efficiencies.
With the system in place, the Turkish government also has data tools in place to support its fight against the selling of narcotics on the black market. The product recall process is also greatly improved. The government can conduct more complex recalls quickly, as the product data is housed in one place. It is now possible for the government or manufacturers to issue a recall for a batch or a half batch of a drug from just the affected stakeholders (vs. the entire drug production or in the whole supply chain).
The recall can be conducted in more specific ways, such as stopping a transaction at the point of sale or following the movement of the recalled product backward in the supply chain. Once a recall is issued in the system, the recall takes effect instantly and no entity can challenge the recall. The product data captured in the system is valuable and can be used to support rational use of medicines, create administrative reports, monitor the industry and prevent tax fraud, among other uses. Using this instantly updated data, faster decisions and more consistent estimates can be made.
Conclusion and Next Steps

The system's Outcomes and benefits have inspired other industries and countries. The Turkish government anticipates applying nationwide track and trace systems for medical devices and food sectors. The Turkish Medicines and Medical Devices Agency recently invested significantly in a project to track and trace medical devices and cosmetics. Also, Türkiye is working on further enhancing its approach to traceability.
A new, innovative project investigates a rule-based approach, which may provide an adjustable and dynamic structure. Such a structure would allow the existing system to be customized concerning various regulations, serialization standards and technologies. With this innovation in place, the system could accommodate any country's regulation and trace any packaged product without needing redevelopment. Furthermore, with this innovation, the system can be harmonized and an interoperable region-wide traceability system.
Türkiye's government and pharmaceutical industry stakeholders are working to increase safety and security in the pharmaceutical supply chain to prevent theft, counterfeiting and other illegal activities and have gained many additional benefits. Türkiye's traceability system can provide a model for other nations looking to establish a central repository for drug information that can be used to meet CoC/CoO goals.
Source:https://titck.gov.tr/