Success Stories
The Revolutionary Impact of Pharmaceutical Track & Trace System
In an era where the authenticity of pharmaceuticals is a matter of life and death, the need for innovative solutions to combat the scourge of counterfeit drugs has never been more pressing. The pharmaceutical industry, a critical component of global healthcare, faces a daunting challenge: ensuring the integrity and safety of its products in a complex and sprawling supply chain. This is where we step in with our solution, the Pharmaceutical Track and Trace System, branded as DrugXafe.
The implications of counterfeit drugs are vast and alarming. Globally, these fraudulent medications not only undermine public trust in healthcare systems but also pose severe health risks, leading to preventable fatalities and exacerbating existing health crises. The World Health Organization highlights the grim reality: thousands lose their lives yearly due to counterfeit drugs, turning a spotlight on the urgent need for robust tracking systems.
Our Pharmaceutical Track and Trace System is more than just a technological advancement; it's a hope for safeguarding public health. By ensuring that every medicine reaching a patient is genuine, this system acts as a critical shield against the dangers posed by counterfeit drugs. It symbolizes a significant stride in public health safety, intertwining technology with the noble cause of saving lives.
This success story delves into the multifaceted impact of counterfeit drugs, the vital role of our system in combating them and how it reshapes the landscape of global health security. We will explore the intricate details of this innovative system, its implementation, challenges and the remarkable benefits it offers to healthcare authorities, professionals, providers, patients and the pharmaceutical industry.

The Impact of Counterfeit Drug Use Worldwide
The use of counterfeit drugs is a major issue that leads to significant health problems and even deaths globally. This situation seriously threatens public health and must be addressed urgently. We can discuss five significant impacts:
- Patients' Lack of Access to Expected Treatment: In critical illnesses where time is of the essence, the inability to access the right treatment can be fatal. According to World Health Organization reports, preventable diseases have led to numerous deaths. For instance, it's reported that counterfeit drug consumption has resulted in between 70,000 to 160,000 deaths from pneumonia alone. This alarming situation highlights the urgent need for more effective global measures to ensure the availability and authenticity of essential medications.
- Harm from Harmful Substances in Counterfeit Drugs: Interpol reports have identified harmful substances like mercury, arsenic, rat poison and cement in some counterfeit drugs. Also, according to NCBI’s article, there is an example of that the potential financial impact of the issue at hand is staggering, with estimates reaching as high as $200 billion. This significant figure underscores the severity of the problem and its wide-reaching implications. The World Health Organization (WHO) reports a troubling statistic: approximately one million individuals lose their lives annually due to the consumption of counterfeit medications. This alarming number highlights a critical global health crisis.A recent incident in September 2022 brought this issue into sharp focus. The WHO issued a warning about four cough syrup brands produced by a pharma company, a company based in India. These products were implicated in a tragic event in Gambia, a country in Western Africa, where they were linked to the deaths of 66 children. Further investigation revealed a disturbing fact about these cough syrups: they contained dangerously high levels of diethylene glycol and ethylene glycol, both toxic substances when ingested. These contaminants were identified as the probable cause of the fatalities, drawing attention to the dire consequences of substandard pharmaceutical practices.
- Economic Losses and Increased Mortality: NCBI's article discusses the global issue of drug counterfeiting, highlighting that nearly 10.5% of worldwide medications are substandard or fake. It emphasizes the economic losses and increased morbidity and mortality due to counterfeit drugs. The Covid-19 pandemic further exacerbated the issue by fueling the demand for certain medicines, leading to an increase in substandard or fake products. It also covers current trends in drug counterfeiting, prevention measures and the roles of various stakeholders in addressing this challenge.
- The Devastating Impact of Counterfeit Medicines: According to OECD and the European Union Intellectual Property Office (EUIPO) report, counterfeit medicines pose a critical threat not only to public health but also to economies and the environment, affecting individuals, companies, and governments globally. One of the most alarming consequences is the substantial harm to public health, with estimates indicating that between 72,000 and 169,000 children may die annually from pneumonia due to counterfeit drugs. Additionally, fake anti-malarial medication is believed to cause around 116,000 deaths each year. This situation demands immediate and decisive action to safeguard health and lives.
Legitimate pharmaceutical companies, particularly those based in the United States, suffer significantly due to these counterfeits, with about 38% of all seized fake medicines infringing on the intellectual property of U.S. firms. This problem also gravely affects other OECD countries, notably Switzerland, Germany, and France, leading to loss of sales and damage to reputations. The economic impact is also substantial. For instance, the European Union loses approximately EUR 1.7 billion in revenue because of counterfeit medicines. Moreover, treating patients who have suffered adverse effects from these counterfeits incurs additional costs.
Counterfeit medicine production contributes to environmental pollution through unregulated and hazardous practices involving potentially toxic chemicals. Lastly, the social implications are profound, with a surge in organized crime and significant job losses in the pharmaceutical sector and related industries. In the EU alone, over 80,000 jobs are estimated to be lost, highlighting the urgency for a coordinated international response to combat this illicit trade.
Effects of Counterfeit Drugs and Drug Policies

The threat of counterfeit drugs is a global crisis with far-reaching effects on individual and public health, as well as on the integrity of pharmaceutical policies and systems worldwide. Counterfeit medications, which may contain incorrect doses, harmful substances or no active ingredients at all, pose a severe threat to patients who rely on them for life-saving treatments. The impact of these fraudulent products is not confined to health risks alone; it extends to undermining the trust in healthcare systems and drug policies designed to protect public welfare.
Navigating this complex challenge requires a multifaceted approach, combining vigilant regulatory frameworks, advanced tracking technologies and informed public awareness. Drug policies play an essential role in this process, laying the groundwork for ensuring drug quality, safety and efficiency. These policies must adapt continually to combat counterfeiters' evolving tactics and integrate technological advancements
In this context, we will explore how effective drug policies bolstered by innovative solutions like our Pharmaceutical Track and Trace System are critical in the fight against the expansion of counterfeit drugs. This exploration will shed light on the significant strides being made to preserve the sanctity of the pharmaceutical supply chain and protect patients worldwide. We have been at the forefront of combating counterfeit drugs through our DrugXafe. This innovative approach enhances patient safety and ensures the integrity of the pharmaceutical supply chain.
Economic Effects and Counterfeiting Prevention
The economic implications of counterfeit drugs and the importance of their prevention are profound and multifaceted. Counterfeit drugs not only pose a serious health risk but also have significant financial consequences. They undermine the revenues of legitimate pharmaceutical companies, leading to lost profits and reduced incentives for research and development. This affects the pharmaceutical industry and the broader economy through job losses and decreased investment in healthcare innovation.
Preventing the proliferation of counterfeit drugs is, therefore, not only a health imperative but also an economic necessity. Effective countermeasures, such as our Pharmaceutical Track and Trace System, play a crucial role. By ensuring the authenticity of medications, such systems help protect the investments of legitimate pharmaceutical companies and maintain the integrity of the healthcare market.
Furthermore, the prevention of counterfeit drugs helps to conserve public health resources that would otherwise be spent on treating complications arising from substandard or harmful medications. This, in turn, contributes to healthcare systems' overall efficiency and sustainability.
The economic effects of drug counterfeiting are far-reaching, impacting both the health sector and the broader economy. Preventive measures are essential for protecting public health, preserving financial stability and fostering continued innovation in the pharmaceutical industry.

What Is DrugXafe?
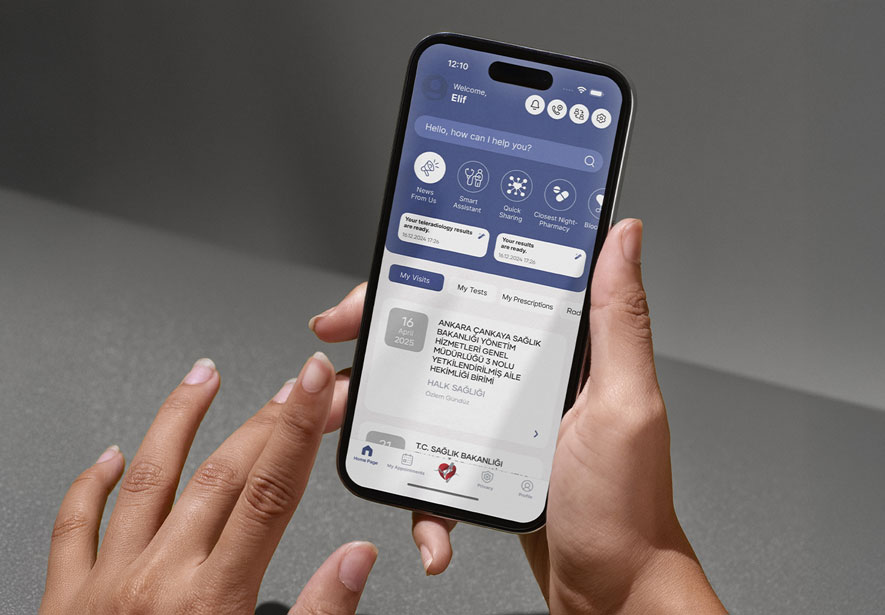
DrugXafe, also known as The İlaç Takip Sistemi (İTS) and the Pharmaceutical Track and Trace System, is a technological advancement in pharmaceutical safety and accountability. As a flagship initiative primarily spearheaded in Türkiye, it is a robust model for global drug tracking and tracing. The essence of İTS lies in its capacity to uniquely identify and track each medication package from production to consumption, ensuring the authenticity and safety of pharmaceuticals in the supply chain.
DrugXafe operates on a sophisticated platform where every stakeholder in the pharmaceutical industry, from manufacturers to pharmacies, is integrated. Each drug package is marked with a unique identifier (2D Data Matrix), then tracked through every stage of its journey. This system allows for real-time monitoring and data collection, providing invaluable insights into drug distribution patterns and usage.
The implementation of DrugXafe marks a significant milestone in combating counterfeit drugs. DrugXafe plays a critical role in safeguarding public health by ensuring that only certified, safe medications reach the end-user. DrugXafe is not just a tracking tool; it's a testament to the power of technology in enhancing drug safety, fostering trust in healthcare systems and ultimately protecting lives.
Besides, this system has a mobile application, as well. DrugXafe Mobile App is an innovative solution designed to empower individuals with immediate access to essential medication information, side effect notification, expiration date and nearest pharmacy. This user-friendly application allows users to quickly scan the data matrix on their medicines, providing them with accurate and details, regardless of their location and time.
Transitioning to Practical Solutions: The Benefits of DrugXafe
Now, let's delve into the specific features of these innovative solutions. Understanding the benefits of DrugXafe is crucial to appreciating their impact on patient safety and supply chain integrity. By examining these benefits one by one, we gain a clearer view of how these systems contribute to the global fight against counterfeit drugs.
Access to Genuine Medication
The importance of patients' access to authentic, safe and effective medication cannot be overstated. In markets with central control, distinguishing between counterfeit and genuine drugs is easier. While some countries attempt to use holograms on drug packaging, their effectiveness is not guaranteed. The ideal solution is the unique identification of each drug package and its monitoring through a centralized system.
Global Health Security
By implementing DrugXafe, we contribute significantly to global health security. This system provides a robust framework for monitoring drug distribution, ensuring that only authentic and safe medications reach patients.
Counteracting Drug Counterfeiting
Our system plays a key role in identifying and eliminating counterfeit drugs from the market. This not only saves lives but also protects the reputation of pharmaceutical manufacturers.
Economic Benefits
By reducing the circulation of counterfeit drugs, the system indirectly contributes to the national economy. It protects pharmaceutical industry investments and ensures funds are channelled towards genuine healthcare development.
The National Economic Impact of Counterfeit Drugs and the Role of DrugXafe
Counterfeit drugs pose a significant threat to public health and the economy. The primary concern is the severe impact on patient health, leading to ineffective treatments, increased healthcare costs and a waste of time and resources. Moreover, counterfeit medications undermine the efforts and investments of legitimate local pharmaceutical producers, damaging the industry's integrity and financial stability.
Stock Management with DrugXafe
Implementing DrugXafe can transform stock management in pharmacies and hospitals. It ensures authentic medications are stocked, reducing counterfeit drug risks. This protects patient health, optimizes inventory, saves costs and increases efficiency. DrugXafe's accurate stock management protects pharmaceutical investments, boosting the healthcare sector's economic stability.
Effective Recall with DrugXafe
DrugXafe's tracking system is crucial for efficient medication recalls. It swiftly identifies and removes specific drug batches, reducing patient health risks and preventing panic. This targeted approach maintains public trust in pharmaceuticals. It contains economic losses from broad recalls, highlighting the significance of strong safety measures in the industry for public health and financial stability.
Reporting Side Effects
Patients can report side effects of drugs to the ministry through a mobile application. Reporting side effects, laboratory investigations and block & recall processes are critical in drug safety.
Data-Driven Decisions
The system facilitates the collection and analysis of vast amounts of drug distribution and usage data. This data helps formulate effective health policies and identify potential outbreaks or health crises.
Strengthening Regulatory Compliance
DrugXafe aids regulatory bodies in enforcing drug safety standards. Providing transparent and accurate data ensures that pharmaceutical companies adhere to legal and ethical practices.

DrugXafe Implementation Process
The Pharmaceutical Track and Trace System (PTTS) in Türkiye, launched between 2009 and 2010, represents a significant advancement in ensuring medication safety and combating counterfeit drugs. This initiative by the Ministry of Health brought together various stakeholders in the pharmaceutical supply chain, from manufacturers to pharmacies. This system's cornerstone is assigning a unique identifier to each medication package, facilitating real-time tracking and verification.
The system's design drew from international best practices but was customized to align with Türkiye's specific healthcare and regulatory requirements. This thoughtful integration has led to DrugXafe setting a new global standard for pharmaceutical safety.
In recognition of its effectiveness and innovation, PricewaterhouseCoopers (PWC), an esteemed independent organization, honored the Turkish Track and Trace System as the 'Best Practice' in the pharmaceutical industry in 2015. Further, in 2016, the World Summit on the Information Society (WSIS) acknowledged DrugXafe as one of the top 5 projects in e-Health worldwide.
The success of DrugXafe in Türkiye has not only been a domestic success but has also had international repercussions. The model's effectiveness inspired other nations to adopt similar systems. For instance, the Saudi Drug Track & Trace project, influenced by the Türkiye system, was recognized with the prestigious worldwide Championship Award in e-Health by WSIS in 2020. This global recognition underscores the far-reaching impact and demand for the DrugXafe solution.
Following Türkiye's lead, the creators of DrugXafe, Tiga Healthcare Technologies, implemented this system in Saudi Arabia in 2018 and Qatar in 2023. As a result, all medications in these countries are now monitored using the DrugXafe system, demonstrating the global demand for and effectiveness of this approach. The Saudi WSIS award serves as a further testament to the system's excellence and its vital role in transforming pharmaceutical safety and tracking on a global scale.
Data Transmission
The Data Transmission aspect of the DrugXafe, is a critical component that ensures the effectiveness and reliability of the entire system. Central to DrugXafe’s success is its ability to handle real-time data transmission. This includes manufacturers, wholesalers, pharmacies and healthcare providers. The system's sophisticated data transmission infrastructure allows for the continuous and secure exchange of information regarding each drug's journey from production to patient. It facilitates tracking unique identifiers assigned to each medication package, ensuring accurate and timely monitoring. This capability helps prevent counterfeit drugs from entering the supply chain and enables quick responses to any irregularities, such as recalls or quality issues. Moreover, the advanced data transmission technology of DrugXafe plays a critical role in gathering valuable data analytics, contributing to better health policy decisions and improved patient safety measures. The precision and efficiency of data transmission in the DrugXafe highlight its importance as a cornerstone in modern pharmaceutical safety and regulatory compliance.
Technical and Managerial Challenges
Implementing a comprehensive pharmaceutical system like the DrugXafe presents a unique blend of technical and managerial challenges. Technically, the primary challenge lies in creating a robust and scalable system that can handle vast amounts of data securely and efficiently. This requires advanced software development, rigorous testing and continuous updates to keep pace with technological advancements and emerging threats, such as cybersecurity risks.
Another significant technical hurdle is ensuring system interoperability. The system must seamlessly integrate with various existing platforms used by different stakeholders in the pharmaceutical supply chain, including manufacturers, distributors, pharmacies and healthcare providers. This integration facilitates a comprehensive and interoperable system for tracking, tracing and managing pharmaceutical products, ensuring seamless oversight from production to consumption.
Managerially, the challenges are equally daunting. One of the most significant issues is stakeholder engagement and coordination. Successfully implementing a system like DrugXafe requires the collaboration and commitment of diverse participants, each with their own interests and operational frameworks. Achieving consensus among all these parties and maintaining their active participation is complex.
Additionally, there's the challenge of compliance and regulation adherence. The system must meet current regulatory standards and be adaptable to future legislative changes. This requires a deep understanding of both local and international laws and standards.
Finally, training and user adaptation are critical managerial aspects. Ensuring that all participants in the system are adequately trained and comfortable with the technology is essential for its success. This involves developing comprehensive training programs and ongoing support systems to assist users in adapting to the new system.
In summary, while the technical challenges are focused on system development and integration, the managerial challenges revolve around stakeholder coordination, compliance and user training, all of which are essential for successfully implementing and operating a comprehensive pharmaceutical tracking system. This is where we come in. We tell you how DrugXafe became a success story by overcoming the abovementioned challenges.

Legal Regulations and Compliance Processes
DrugXafe is closely intertwined with stringent legal regulations and compliance processes, ensuring its effectiveness and alignment with international standards. The legal framework surrounding DrugXafe is designed to uphold the highest standards of pharmaceutical safety, authenticity and traceability. This involves setting comprehensive guidelines for all stakeholders in the drug supply chain, from manufacturers to pharmacies, to adhere to the system's protocols. The rigorous compliance processes involve regular audits and checks to ensure that every participant in the supply chain conforms to the set regulations. These standards align with global best practices, including adherence to GS1 standards for product identification and data sharing. This legal and compliance structure enhances the system's efficiency and fosters a culture of transparency and accountability within the pharmaceutical sector. By integrating these legal and compliance requirements, DrugXafe ensures that the drugs in the market are safe, authentic and traceable, thereby protecting public health and maintaining the integrity of the pharmaceutical industry. The success of DrugXafe in this regard serves as a benchmark for other nations looking to implement similar drug tracking and safety mechanisms.
Data Security and Privacy
Data security and privacy are critical in pharmaceutical systems like the DrugXafe. Protecting sensitive health and proprietary data requires robust encryption and strict access controls. These systems must adhere to stringent data protection regulations, such as KVKK in Türkiye, GDPR in Europe or HIPAA in the United States, ensuring that patient and drug data are handled with the utmost confidentiality and integrity. Regular security audits and updates are crucial to safeguard against cyber threats.
Additionally, educating stakeholders about data privacy practices is essential. The balance between accessibility and security is key, ensuring that while the system remains efficient and user-friendly, the data within is shielded against unauthorized access, breaches and misuse. Security measures at the user, stakeholder and system levels were implemented to prevent unauthorized access. Personal data is encrypted within the system.
System Installation Phases
DrugXafe installation is a comprehensive process methodically executed in several vital phases to ensure its efficiency and effectiveness. The initial phase involves meticulous planning and design tailored to meet the specific requirements of the Turkish pharmaceutical sector. This includes identifying the system's objectives, scope and stakeholders, along with establishing the necessary infrastructure.
The subsequent phase in software development places a significant emphasis on crafting a system that is not only user-friendly but also inherently adaptable and scalable. This approach ensures that the development process is tailored to meet the demands of both today and the future. Designing a system that can evolve and expand effectively is essential, as it underpins the ability to handle varying volumes and complexities of data efficiently and securely.
After successful testing, the system undergoes a pilot phase where it is implemented in a controlled environment to assess its functionality and gather feedback for any needed improvements.
The final phase involves the full-scale deployment of DrugXafe across the entire pharmaceutical supply chain. This includes the integration of all stakeholders, from manufacturers to pharmacies and the continuous monitoring and refinement of the system. Training and support are provided to ensure smooth adoption and usage.
Throughout these phases, emphasis is on the centre due to the compliance with legal requirements, data security and system reliability to establish a trustworthy and effective pharmaceutical tracking system.
Impact Analysis
DrugXafe reveals significant positive outcomes in enhancing pharmaceutical safety and combating counterfeit drugs. Central to its success is the system's ability to accurately trace the journey of each medication from manufacturer to consumer, thereby ensuring drug authenticity and patient safety.
Key impacts include a marked reduction in the prevalence of counterfeit drugs in the market, directly translating to improved public health outcomes. The system's real-time tracking capabilities have also enabled quicker responses to drug recalls and quality issues, safeguarding patient health.
Economically, DrugXafe has bolstered the pharmaceutical industry by protecting the investments of legitimate drug manufacturers and distributors and fostering consumer trust in pharmaceutical products. Additionally, the system's comprehensive data collection has provided valuable insights for healthcare policy and decision-making, enhancing the overall efficiency of the healthcare system.
The DrugXafe has also set a precedent in regulatory compliance, establishing a model for other countries to follow in pharmaceutical safety and tracking. Its implementation demonstrates the significant role of technology in healthcare and the potential for similar systems to make a global impact.

Future Projections and Expansion of DrugXafe
- Adapting to Emerging Challenges: As the pharmaceutical industry evolves, we are ready to update and expand DrugXafe to meet new challenges continually. This includes integrating advanced technologies for enhanced security and traceability proactively.
- AI Integration: Upcoming developments include the integration of AI. This advancement will provide predictive analytics for drug usage patterns, potential shortages and outbreak predictions, enabling proactive healthcare management.
- Collaboration with International Bodies: We seek to collaborate with international health authorities, organizations and pharmaceutical bodies to standardize drug tracking globally. This initiative will ensure a unified approach to tackling the issue of counterfeit drugs worldwide.
Our Role in the Global Health Sector

Our role extends beyond technological innovation; it is reshaping how the world approaches drug safety and integrity. By providing a reliable solution to combat counterfeit drugs, we significantly contribute to ensuring that medications are safe and effective for patients worldwide.
Our system enhances the transparency and security of the pharmaceutical supply chain, offering a model that can be adapted and adopted globally. This strengthens healthcare systems and builds public trust in pharmaceutical products.
Furthermore, the data collected through DrugXafe offers invaluable insights for health policy development and epidemic surveillance, contributing to improved public health outcomes.
- Setting Industry Standards: Through our innovative solutions, we are setting new standards in the pharmaceutical industry for drug safety and traceability. Our systems serve as a model for other companies to follow.
- Contributing to Public Health: By ensuring the authenticity of drugs, we play a crucial role in public health. Our system helps prevent the devastating effects of counterfeit drugs on communities.
- Promoting Transparency: Our Pharmaceutical Track and Trace System supports transparency in the drug supply chain, building trust among consumers, healthcare providers and regulatory bodies.

DrugXafe represents a monumental leap forward in the ongoing struggle against counterfeit drugs. This innovative system embodies the cutting-edge technology in the pharmaceutical sector and serves as a testament to the power of dedicated efforts in safeguarding public health. By meticulously tracking and verifying the authenticity of pharmaceuticals, our system ensures that patients receive genuine, effective medication, thereby significantly reducing the risks associated with counterfeit drugs.
The broader implications of this system extend far beyond individual patient safety. It's a crucial tool in upholding the integrity of the global pharmaceutical supply chain, bolstering public trust in healthcare systems and contributing to the overall well-being of communities worldwide. Economically, it safeguards investments in the pharmaceutical industry, ensuring that resources are channelled towards legitimate and beneficial healthcare developments. Furthermore, by setting a high standard for regulatory compliance, DrugXafe encourages an industry-wide shift towards greater transparency and accountability.
DrugXafe is not just a solution to a problem; it's a hope of innovation and trust in the healthcare IT. As we look towards the future, the system's potential for adaptation and integration with emerging technologies like AI presents new horizons for enhancing global health security. Our commitment to excellence and innovation in this field is a shining example of how technology and foresight can converge to create a safer, healthier world for all.
Let's shape the future together!