Aggregation Management System
The Aggregation Management System identifies each product's serial number in a package with the main serial number for that given package. It groups product units with multiple serial numbers. Forming parent-child associations ensures easier tracking and tracing of the box, package and pallet-based drug movements throughout every stage of the supply chain.
Benefits and Innovations
The Aggregation Management System complements the Pharmaceutical Track & Trace System in the packaging and logistics management layer. It processes the information of the packages in which each medicine box is placed and the pallets containing these packages into barcodes. The Pharmaceutical Track & Trace System uses this information to track and trace drugs on a box-package-pallet scale.
Benefits
- Advanced Drug Safety
- Clean and Transparent Supply Chain
- Effective Drug Recall Process
- Reduced Human Errors and Interventions
- Seamless Inventory Management
- Efficient Warehouse Management
- Sustainable and Efficient Resource Utilization
- Ensuring Time Savings
- Complying with the Regulations
- Significant Decrease in Costs and Workload
Innovations
- Serialization
- Full Visibility
- Aggregated Manner of Supply Chain
- Big Data for Decision-Makers
- Sustainable and Scalable Supply Chain
Pharmaceutical Track and Trace at Box-Package-Pallet Scale:
Aggregation Management System
The system, which provides access to drug information on drug boxes, packages and pallets, enables the collective tracking of the drug supply chain. It also streamlines the track and trace process, ensuring efficiency, time savings and accuracy in pharmaceutical supply chain management.
Features
Provides Drug Safety
DrugXafe employs a sophisticated 2D Data Matrix at the onset of production and generates new SSCC (Serial Shipping Container Code) for the package and pallet, acting as a robust barrier to infiltrating counterfeit drugs into the system. This barcode system contains the Global Trade Item Number (GTIN), Serialization Number (SN), Expiration Date (XD) and Batch Number (BN). This innovative approach ensures the authenticity and safety of drugs from the production stage.
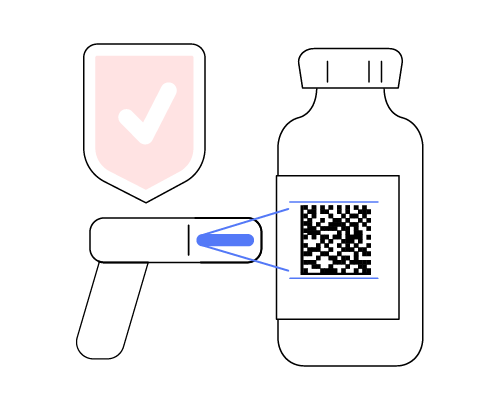
Clean Supply Chain
Assigning a unique 2D Data Matrix code to every drug package and palette effectively serializes each one. DrugXafe meticulously records the journey of every drug, from the producer and importer to the patient, across all stages. This comprehensive tracking mechanism effectively prevents the infiltration of counterfeit drugs into the supply chain, ensuring its integrity and cleanliness. Through this process, DrugXafe contributes significantly to safeguarding public health by guaranteeing that only genuine, safe medications reach consumers.
Package and Palette Level Tracking & Tracing
Integrated with our Pharmaceutical Track and Trace System, branded as DrugXafe, the Aggregation Management System tracks and traces high-volume product movements in the supply chain, including production and storage. It generates new SSCC for the package and pallet in which it is placed and updates stakeholders like warehouses, pharmacies and healthcare facilities, ensuring seamless track and trace capability.
Regulatory Compliance
The 2D Data Matrix on each drug package and palette ensures serialization and regulatory compliance, enabling complete tracking throughout the supply chain. Starting from regulations and legislation, the system prioritizes drug integrity and safety, upholding the highest data privacy and clean supply chain management.
Inventory Management
This system contributes to effective inventory management at the pharmaceutical production facility and warehouse level. Real-time tracking and tracing ensures optimized inventory management capabilities to meet changing demands and product conditions.
Product Recall
The Aggregation Management System facilitates the withdrawal of recalled drugs from the market at the box, package and pallet levels. The system updates all stakeholders according to recall decisions taken by health authorities and regulatory bodies. It closes drug sales or suspends their sales. Thus, drug recall processes are managed quickly and effectively.
Flexible Supply Management
The aggregation Management System allows flexible control over the pharmaceutical supply chain and it quickly adjusts to changing pharmacy and warehouse demands. It facilitates easy increases or decreases in the number of medicines ready for delivery, ensuring efficient resource allocation. This optimization minimizes errors and maintains optimal inventory levels.
Real-Time Data Management
Real-time control of drug packages and pallets ensures immediate visibility into product movements. This feature enables stakeholders to track and trace product conditions, shipments, inventory and purchase status. Facilitating data sharing improves decision-making and safety across the supply chain.
Batch Number (BN)
The Batch Number (BN) feature within the Aggregation Management system is crucial for ensuring the traceability and authenticity of pharmaceutical products. By uniquely identifying each batch, the BN facilitates efficient tracking and management, significantly reducing the risk of counterfeit products entering the supply chain. This precise identification aids in swiftly addressing quality control issues, ensuring the highest safety and reliability standards in drug distribution.
Benefits for Patients
Enhanced
Patient Safety
Patients are protected from counterfeit and substandard drugs, ensuring safe medication usage.
Effective
Recall
The risk of using recalled medicines is reduced with the help of effective recall management.
Expiry Date
Alerts
The system tracks the expiration date of the medication and prevents the patient from accessing an expired medication.
Trustable
Supply Chain
Patients are assured that their medicines come from a secure pharmaceutical supply chain.
Benefits for Healthcare Systems, Providers and Authorities
Clean
Supply Chain
DrugXafe blocks fake medicines, ensuring a clean supply chain with serialization.
Regulation
Compliance
Ensures that this process is carried out by the legislation in a harmonized manner.
Seamless
Traceability
Ensures real-time tracking and tracing of the origins and pathways of drug packages and palettes.
Fraud
Prevention
Authorities prevent drug fraud, safeguarding the integrity of the pharmaceutical market.
